Medical Research by
Dr. Syed RehanIftikharBukhari Prof.Dr.IstvánBerkes
Injuries to the hyaline cartilage (vitreous cartilage) covering the bony surface of joints are becoming more common in athletes. Their treatment is a major challenge, mainly because of the low regenerative potential inherent in the specific characteristics of cartilage tissue. According to literature, vitreous cartilage injuries are twice as common in athletes as in the non-athlete population. They typically occur more frequently in competition than in training and in people with a body mass index above 30. Professional footballers are at 12 times the risk of severe cartilage damage.
The vitreous cartilage covering the surface of joints is characterised by its elastic resistance to pressure, elastic strength and low bending capacity. The vitreous cartilage is composed of cartilage cells and an intercellular matrix of collagen fibres and proteoglycans (glucosamine, chondroitin sulphate) produced by the cartilage cells. The hyaline cartilage contains neither blood vessels nor nerves. It is nourished by diffusion.
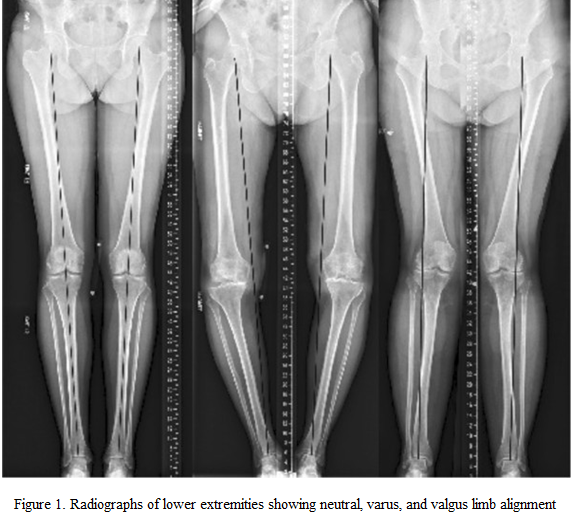
Cartilage lesions may be caused by metabolic stimulation, genetic or vascular events, and more commonly by chronic overload and less commonly by traumatic injury. Anatomical abnormalities (e.g. O-leg, X-leg) and joint instability are the common risk factors. The main problem in terms of treatment is that the damaged vitreous cartilage is unable to regenerate spontaneously due to a lack of blood supply. The pain that occurs when cartilage is damaged and lacks nerve supply can be explained by the fact that the tissues near the cartilage, such as the synovium and the underlying bone, have an abundant nerve supply and that the inflammatory substances released after cartilage damage and the direct high loads on the bone stimulate the nerves. As synovial fluid is vital for cartilage cells, any prolonged disturbance of the joint cavity (haematoma, acid, or pus) can cause cartilage cell death. Due to the low nutrient and oxygen requirements of cartilage cells, no cell death occurs in cartilage fragments removed during surgery even after 24-48 hours of transport, so it is possible to culture large quantities of living chondrocytes from cartilage fragments sent to the laboratory for chondrocyte implantation.
Diagnostics
The typical symptoms of a chondral lesion are pain on exertion, cracking, clicking, jamming, joint locking and joint swelling during movement, which together may cause inability to exercise. Up to 14% of athletes may be asymptomatic, even with full thickness defects. An accurate diagnosis of cartilage injuries requires careful history taking, thorough physical and X-ray examination. More detailed detection of lesions is possible with magnetic resonance imaging (MRI). Contrast-enhanced and new functional MRI techniques also provide insight into the biochemical and biomechanical status of cartilage and sub-cartilage bone. The most accurate pathology can be obtained by arthroscopy. Cartilage injuries can be classified into 4 stages depending upon their location, size and thickness according to the international ICRS classification. Stages 1-2 (softening, cracking) can usually be treated conservatively, while the stages 3-4 (fragmentation, ulceration) surgically. It is important to note that, with modern non-invasive examination facilities, the use of arthroscopy for diagnostic purposes only is not acceptable, but should be used to assess lesions that have already been diagnosed more accurately and to perform the appropriate surgical intervention. “Peeking” and/or flushing the joint without an accurate diagnosis is also contraindicated, as it can cause serious complications.
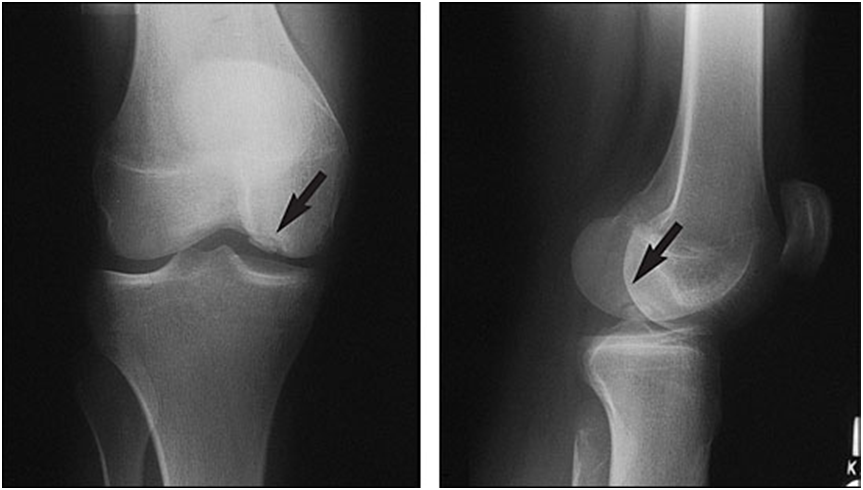 Figure 2. Radiographs showing osteochondral lesion of the medial femoral condyle
Figure 2. Radiographs showing osteochondral lesion of the medial femoral condyle
Conservative treatment
Initially, the treatment of cartilage injuries is conservative, which means adjusting the body weight as necessary, modifying sports activities, cooling, strengthening muscles, special gymnastics, and taking painkillers and anti-inflammatory drugs. However, efforts should be made to ensure that the injured person is as physically active as possible and to promote this, for example through cross-training. The use of various oral cartilage regeneration drugs and cartilage support supplements is also recommended as part of the therapy. The active substances in these preparations are glucosamine, chondroitin sulphate and hyaluronic acid, which are important building blocks of cartilage tissue. All three improve the water retention capacity of cartilage, thus helping the articular surfaces covered by cartilage to glide, and inhibit the enzymes that break down cartilage, as well as having analgesic and anti-inflammatory effects. Their effectiveness is not sufficiently proven. They may slow down the process of cartilage degradation and reduce the symptoms of the disease, but they cannot regenerate cartilage tissue and form new cartilage. Since their use has no known adverse effects and they produce positive subjective results, they can be recommended as a course of treatment according to the following schedule: after 2 months of use, a break of 1 month, followed by another 2 months of use, which can be repeated 2 times a year. A doctor’s recommendation is essential.
When oral medication is no longer sufficient to reduce the symptoms, intra-articular injections can be used. The most commonly used globally are the steroid injections, which effectively reduce pain by stopping inflammation. However, their use requires great caution, even with slow-absorbing drugs, because of the well-known side effects (lowering of immunity, increase in blood sugar and blood pressure for 2-4 weeks after injection, long-term cartilage damage). They are recommended to be administered 1 time per week, repeated up to three times.
Injections with hyaluronic acid, the so-called “glide-enhancing” active substance, have far fewer side effects. They do not usually have an immediate effect, but rather a slower but more prolonged effect. Their real effectiveness is questionable, but they also reduce inflammation to some extent, in addition to facilitating gliding. They are usually recommended to be repeated 3-5 times, usually weekly. They can be an alternative to surgery or at least delay it in time.
Biological preparations derived from naturally occurring substances in the body, such as platelet-rich injections (PRP therapy), bone marrow concentrates and stem cell injections from various tissues, are increasingly used to treat cartilage damage. The use of PRP therapy and bone marrow concentrate is safe and the results are encouraging. They reduce symptoms, but their tissue regenerative effects are not proven. When combined with surgical intervention, they offer significant benefits. Stem cell therapy by injection into the joints is still in the experimental stage.
Surgical treatment
Surgical intervention is justified in case of deterioration of the joint condition, appearance of mechanical symptoms or failure of conservative treatment. Since the most important aspect for athletes is a safe and early return to sports, the joint decision-making and treatment algorithm related to surgery must be subordinated to this. Special athlete needs and multifactorial parameters require an individualized approach.
The heterogeneity of patients and cartilage injuries significantly influences the treatment decision-making process. A 2 cm2 cartilage damage in a 1.65 m tall football player may require a different approach than a 2 m tall basketball player with the same size damage, even in the same anatomical location. The 2 cm2 lesion of the medial trochlea of the femur has different symptoms than the 2 cm2 defect of the lateral trochlea. Cartilage damage to the lateral femoral condyle of the knee requires different treatment depending on the extent of the subchondral lesion. Previous surgeries (e.g. meniscus removal) also affect the treatment.
For athletes, the timing of treatment is also a key factor. In the case of an athlete who has been complaining for more than 1 year, returning to sports is an unrealistic expectation. Athletes with mild symptoms may be able to defer treatment in certain circumstances, such as due to a contractual obligation or seasonal challenge, due to their role in a team. The shared decision-making process should focus primarily on the athlete, but others, such as family members, coaches, team leaders,and managers, should also be involved, and individual expectations should always be taken into account.
Younger age (<25 years for competitive athletes, <30 years for recreational athletes), traumatic injury, diagnosis and treatment within 1 year of symptom onset, minor cartilage damage (<2 cm2), no previous surgery or injury and adherence to rehabilitation protocol are all factors that are favourable for successful outcome and return to sport. Untreated cartilage damage or cartilage damage older than 1 year or initially treated with bone marrow stimulation techniques has the worst outcomes.
Surgical intervention with a preventive indication may also be recommended in cases where the particular cartilage lesion or its underlying biomechanical cause, such as axial deviation of the lower limb, leads to rapid progression of cartilage loss.In cases requiring surgical intervention, 3 types of solutions (symptomatic, regenerative, reconstructive) are possible, which can be performed arthroscopically or open. However, the aim should always be to minimise invasiveness.
Symptomatic surgery
Debridement, chondroplasty: Its essence lies in the elimination of harmful enzymes, the removal of inflammatory tissues and the smoothing of surface irregularities, the removal of unstable pieces of cartilage, and the smooth and stable formation of the edges of the defect. It is recommended for athletes with partial thickness, smaller lesions (2-3 cm2), exposed to less stress, as a temporary solution during the competition season or at the end of their sports career. It is considered as a symptomatic treatment, and safe intervention, with relatively quick rehabilitation and return to sports, at low cost. However, its disadvantage is that it does not restore the normal structure and function of the cartilage, and provides only a short, temporary effect on its own. Arthroscopic debridement is still a widely popular procedure today. It is performed during almost all arthroscopic surgeries in preparation for further interventions.
Regenerative surgery
Bone marrow stimulation with microfracture:Its essence is to provide access to the hard-to-heal cartilage defects for the stem cells in the bone marrow. To do this, the layer of bone under the cartilage must be punctured with a pointed saw in several places, perpendicular to the surface, and a well-bleeding, large surface must be formed. Through the holes created 3-4 mm apart, the place of cartilage defect is first filled by a blood clot, then by an organic scar and finally by fibrous cartilage. The method requires 6 weeks of off-loading, then 4 weeks of partial loading with crutches, followed by a gradual transition to full loading. A surgical procedure often used to treat small, i.e. less than 2 cm2, full-thickness bony-cartilaginous defects in mostly less physically demanding patients. The final result depends on the size of the defect. It produces acceptable results in the short term, but based on long-term tests, the quality of the newly formed fibrocartilage is weaker and more fragile than the original vitreous cartilage from a biomechanical point of view, and it can wear out again in a few years. Due to its technical simplicity and the ability to perform it arthroscopically, this is the most commonly used cartilage resurfacing procedure today. Its effectiveness can be increased by adding concentrated bone marrow aspirate or peripheral stem cells, the use of which provides the opportunity to treat larger defects and improves the quality of the formed cartilage.
Reconstructive surgery
Bone-cartilage autograft transplantation (mosaicplasty): The procedure, commonly known only as mosaicplasty – the development of which is attributed toLászlóHangody – is based on the transplantation of small bone-cartilage cylinders extracted from the non-load-bearing surface of the joint to the place of cartilage deficiency. The implanted bony-cartilaginous cylinders are integrated into the receiving bone, and after half a year, the cartilage covering of the joint appears to be completely intact. It is used to replace circumscribed, small and medium (1-2.5 cm2) bony-cartilaginous defects. Compared to the bone marrow stimulation method, the basic advantage of mosaicplasty is that the cartilage formed here fully meets the requirements of vitreous cartilage histologically. The disadvantage of the method is that since the cylinders are removed from the joint itself, complaints may develop at the place of removal, and the number of available cylinders is limited, so it cannot be used to replace larger areas. Donor site complaints can be prevented with plugs made of materials similar to the osteochondral allograft placed in the extraction holes. During the post-treatment, movement favourable to the nutrition of the cartilage surface is allowed immediately, but 3-4 weeks of full or partial weight bearing is required. In 6-8 weeks, the patient can return to his normal everyday activities, and depending on the size and location of the defect, sports activities can also be performed after 4-6 months.
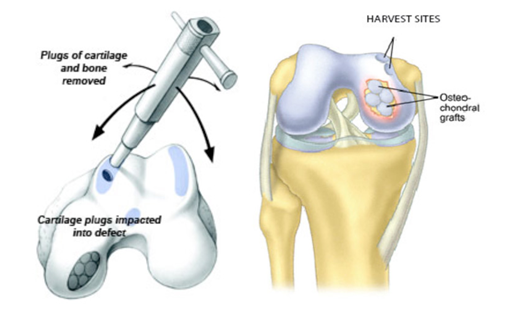
Figure 3. Pictorial representation of osteochondral graft transfer by mosaicplasty
Osteochondral allograft implantation: Its essence is to replace the osteochondral deficiency with joint material sterilely removed from a cadaver, i.e. a human donor, properly stored, inserted to the required size and properly fixed. Recommended procedure for the replacement of large (> 2 cm2) cartilage defects in typically young, active patients exposed to heavy loads. The risk of developing an immune reaction against foreign tissue (e.g. rejection) does not occur in the case of cartilage, and is rare in the case of cleaned bone. Transmission of infection (hepatitis, HIV virus) is often thought to be a greater danger for patients, which can only occur in exceptional cases with the currently used detection methods. The chance of transmitting the virus is 1:1,000,000 or maybe even less. The results are not perfect every time, as the implanted tissue may die and require another operation. The integration of the implanted graft and the growth of the cartilage can be improved by using a concentrated bone marrow aspirate. And here we can also mention the possibility of using minced juvenile allograft cartilage.
Own cartilage cell implantation (autologous chondrocyte implantation (ACI): A two-stage procedure used to replace medium and large (2-10 cm2) full-thickness cartilage defects without the need to remove a larger sample from the joint. The point is that during an arthroscopic intervention, a piece of vitreous cartilage the size of a grain of rice is removed from the non-load-bearing area of the joint, which is sent to the laboratory for culture. This is followed by 4-8 weeks of cultivation in the laboratory, and when the appropriate number of cells has been formed, during a second operation, the solution containing the cells is injected with the help of a small injection needle under the flap (periosteum or artificially produced collagen membrane) sutured onto the cartilage, where cartilage cells adhere, and new cartilage is created in place of the lack of cartilage. Hypertrophy of the periosteal patch or cell leakage can occasionally be a problem.
Matrix autologous chondrocyte implantation (MACI):An improved version of the circumscribed cartilage defect replacement. It involves harvesting three grains of articular cartilage, about the size of a grain of rice, during an arthroscopy and sending them to a laboratory for culture. The cartilage cells, cultured in the laboratory for 4-8 weeks, are plated onto a polymer (matrix, scaffold) that forms a three-dimensional mesh, and then cultured for a further 1-2 weeks. The cartilage cells adhere to the matrix, and the cartilage matrix starts to produce cartilage in the laboratory. In a second operation, after cleaning and preparing the cartilage defect area, a polymer mesh containing the cells is placed on the surface of the cartilage fossa. The polymer that provides the mesh is absorbed into the joint and replaced by the intercellular cartilage. In the operating theatre, the surgeon can further shape and cut the sample to size. The specimen is fixed in place by adhesive (tissue adhesive), suture or other bone fixation methods, while maintaining constant moisture.
After the above two operations, weight-bearing with immobilization for 6 weeks and then rehabilitation according to a strict protocol is required. Recovery of original function is expected after 6 months. The disadvantage of this type of surgery is that two surgical procedures are required and the resulting vitreous cartilage is not fully fibrous and does not correspond to the original vitreous cartilage. However, the biggest obstacle to its wider uptake is that the laboratory labour costs for cell culture are extremely expensive, ranging from €3-4,000 in the first case to €6-8,000 in the second.
Biodegradable technologies
They are designed to enhance the biological response to microfracture and improve cartilage formation. During the procedure, a scaffold, usually an allograft scaffold, prepared in the laboratory, forming a three-dimensional mesh, containing frozen cartilage cells, chondrogenic growth factors and intercellular matrix proteins, hydrated with platelet-rich plasma, is cut to the size of the cartilage defect and placed in the microfracture-prepared area, then fixed with sutures or anchors, possibly with fibrin glue. Post-tests show that the cartilage formed results in a better collagen profile compared to microfracture. The main advantage of these procedures is that they are effectively a one-step surgical option.
Additional surgeries
The treatment of pathological conditions associated with cartilage injury, such as anterior cruciate ligament deficiency, patellar instability, meniscal tears or limb axis misalignment, is as important as the treatment of cartilage.And if, despite all efforts, extensive degeneration of the joint and ultimately arthrosis develops, an endoprosthetic solution, i.e. implantation of a prosthesis, may be recommended. However, after prosthesis surgery, only sports activities that do not cause pain or swelling or impose a high repetitive strain on the joint, such as walking, swimming and cycling, are recommended. Higher loads carry the risk of premature wear of the prosthesis.
Rehabilitation
Aims to relieve clinical symptoms, restore pain-free, full range of motion and muscle strength without increasing cartilage degeneration. A successful outcome requires open communication between the rehabilitation team and the surgeon. Lack of individualised and criteria-based treatment and premature return to sport can lead to persistent pain and further deterioration.Rehabilitation includes physiotherapy exercises, electrotherapy, strength, balance, and endurance trainings. It is well-focused towards pain management, improved mobility and range of motion (ROM) in the initial phases; and the improvements in the strength, power, endurance, stability, and extensibility of the associated joint structures in the later phase. However, the type, intensity and frequency of these trainings are tailored according to the individual physical and psychological needs. During the acute recovery phase, rehabilitation focuses on careful ROM exercises to accomplish complete joint ranges and a normal gait pattern. The next step is to restore muscle strength. After achieving sufficient strength, the rehabilitation focuses on proprioception and agility training, the aim of which is to improve functional performance.
Afterwards, the focus is to gain sports specific skills with a goal to prepare the athletes for the sports participation. Patients are permitted to gradually participate in their respective competitive sports activities only with certain criteria i.e.: the achievement of at least 90% of quadriceps and hamstring strengths as well as the squat ability, complete joint range of motion, above 90 percent on patient reported outcome scores, joint laxity <3mm, and less than 5% difference in load symmetry and stability index, as compared to the unaffected side. Those who meet the criteria are allowed to return to competitive sports only through sports specific rehabilitation resulting in return to team training, where the patients are trained to achieve maximum confidence and motivation in their sports activities, along with a continued injury prevention program while staying active in team sports.
Results
Small cartilage injuries respond well to microfracture or mosaicplasty. Results can be improved with the use of bone marrow concentrate or mesenchymal stem cells. For medium to large lesions, the results of mosaicplasty or MACI techniques or osteochondral allograft implantation are better than microfracture. In cases of subchondral bone involvement, mosaicplasty, osteochondral allograft implantation and MACI technique provide good results.
A follow-up of the various cartilage resurfacing surgeries found that overall 76% of athletes were able to resume some level of sporting activity, on average 9 months after surgery. After mosaicplasty 93%, osteochondral allograft transplantation 88%, autologous cartilage cell transplantation 82% and microfracture 58% of athletes returned to sport. Mosaicplasty resulted in the fastest return to sport at 5 months, while microfracture required 9 months, bone-patellar allograft transplantation also required 9 months and autologous cartilage cell transplantation required 11 months.
The analysis showed that mosaicplasty was more durable (8 years) than microfracture (4 years). Mosaicplasty also had a lower reoperation rate than microfracture 5 and 10 years after surgery. The failure rate of osteochondral allograft implantation ranged from 8 to 50%. The overall failure rate of the MACI technique was 9.7%.
Prevention
The emphasis on prevention also applies for cartilage injuries in athletes. The most important tasks are maintaining ideal body weight, correcting anatomical and biomechanical abnormalities, proper training load, learning correct sports exercise, achieving the desired strength status and developing good muscle strength, professional treatment of minor injuries, and correct sports nutrition. In the case of more serious cartilage lesions, a change of sport or even the cessation of competitive sport may be justified. For athletes who put extreme strain on their joints (e.g. hammer throwers), the use of cartilage support supplements and injections/ hyaluronic acid may be recommended as a preventive measure.
The future
One possibility for articular cartilage resurfacing is the replacement of vitreous cartilage using pluripotent human stem cells. From these highly proliferative cells found in tissues of the body, almost all types of supporting and moving tissue can be formed under appropriate hormonal, cytokine and external environmental influences. Their extraction is easier than that of cartilage samples. Experiments with stem cells so far are promising, but have not yet succeeded in producing clearly acceptable, high-quality vitreous cartilage.
Cartilage resurfacing by genetic engineering could be the real breakthrough. The idea is to use viruses to introduce the genes responsible for cartilage regeneration into the patient’s joint, where they enter the cartilage cells and induce increased cartilage formation activity, resulting in the formation of new cartilage in place of the damaged cartilage. Research of this kind is still at an experimental stage. A big question is also what the application of artificial intelligence will bring to the solution of cartilage surface injuries.
Take-home message
According to current medical knowledge, cartilage degeneration can be slowed down, but once damaged, cartilage cannot be rebuilt, i.e. cartilage damage cannot be reversed. Optimal treatment of cartilage injuries to the vitreous cartilage that covers the articular surface of the bones that make up the joints of athletes remains a major challenge today, despite the availability of a number of new conservative and surgical treatment options. Consensus on the most effective therapeutic strategy is still lacking. Personalised, sport-specific care tailored to the individual needs of athletes is only possible by following an evidence-based treatment algorithm.
References:
- Frank RM, Lee S, Levy D, Poland S, Smith M, Scalise N, et al. Osteochondral allograft transplantation of the knee: Analysis of failures at 5 years. Am J Sports Med 2017; 45:864-74.
- Gracitelli GC, Meric G, Pulido PA, McCauley JC, Bugbee WD. Osteochondral allograft transplantation for knee lesions after failure of cartilage repair surgery. Cartilage 2015; 6:98-105.
- Briggs DT, Sadr KN, Pulido PA, Bugbee WD. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage 2015; 6:203-7.
- Merkely G, Ackermann J, Farina EM, VanArsdale C, Lattermann C, Gomoll AH. Shorter storage time is strongly associated with improved graft survivorship at 5 years after osteochondral allograft transplantation. Am J Sports Med 2020; 48:3170-6.
- Gross AE, Kim W, Las Heras F, Backstein D, Safir O, Pritzker KP. Fresh osteochondral allografts for posttraumatic knee defects: Long-term followup. ClinOrthopRelat Res 2008; 466:1863-70.
- Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg [Br] 1997; 79:1008-13.
- Hangody LR, Gál T, Szűcs A, Vásárhelyi G, Tóth F, Módis L, et al. Osteochondral allograft transplantation from a living donor. Arthroscopy 2012; 28:1180-3.
- Hangody LR, Gal T, Vasarhelyi G, Hangody G, Bukhari Syed RI, Hangody L. Results of ultra-fresh osteochondral allograft transplantation for large cartilage defects in the knee joint. Jt Dis RelatSurg 2022;33(3):521-530.
- Görtz S, Bugbee WD. Allografts in articular cartilage repair. Instr Course Lect 2007; 56:469-80.
- Van Ginckel A, Verdonk P, Victor J, Witvrouw E. Cartilage status in relation to return to sports after anterior cruciate ligament reconstruction. Am J Sports Med. 2013 Mar;41(3):550-9.
- Canale, S.T.; Beaty, J.H. Campbell’s Operative Orthopaedics E-Book; Elsevier: Amsterdam, The Netherlands, 2012.
- Podlog L, Dimmock J, Miller J. A review of return to sport concerns following injury rehabilitation: practitioner strategies for enhancing recovery outcomes. PhysTher Sport. 2011 Feb;12(1):36-42.
- Ardern CL, Glasgow P, Schneiders A, Witvrouw E, Clarsen B, Cools A, Gojanovic B, Griffin S, Khan KM, Moksnes H, Mutch SA, Phillips N, Reurink G, Sadler R, Silbernagel KG, Thorborg K, Wangensteen A, Wilk KE, Bizzini M. 2016 Consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br J Sports Med. 2016 Jul;50(14):853-64.
- Blanch P, Gabbett TJ. Has the athlete trained enough to return to play safely? The acute:chronic workload ratio permits clinicians to quantify a player’s risk of subsequent injury. Br J Sports Med. 2016 Apr;50(8):471-5.
- Şahin AA, Değirmenci E, Özturan KE, Fırat T, Kükner A. Effects of adipose tissue-derived stromal vascular fraction on osteochondral defects treated by hyaluronic acidbased scaffold: An experimental study. Jt Dis RelatSurg 2021; 32:347-54.
About Authors:
This research article is published by Syed Rehan Iftikhar Bukhari 1, IstvánBerkes1, 2
from Doctoral School of Clinical Medicine, Semmelweis University, Budapest, Hungary & Department of Sports Medicine, University of Physical Education, Hungary.


